An HBsAb-negative employee has a percutaneous exposure to blood from a Hepatitis B surface antigen (HBsAg) positive patient. Which of the following regimens is recommended for this employee?
Immune serum globulin and hepatitis B vaccine
Hepatitis B immune globulin (HBIG) alone
Hepatitis B vaccine alone
Hepatitis B immune globulin (HBIG) and hepatitis B vaccine
Answer:
Explanation:
The correct answer is D, "Hepatitis B immune globulin (HBIG) and hepatitis B vaccine," as this is the recommended regimen for an HBsAb-negative employee with a percutaneous exposure to blood from an HBsAg-positive patient. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, which align with recommendations from the Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP), post-exposure prophylaxis (PEP) for hepatitis B virus (HBV) exposure depends on the employee’s vaccination status and the source’s HBsAg status. For an unvaccinated or known HBsAb-negative individual (indicating no immunity) exposed to HBsAg-positive blood, the standard PEP includes both HBIG and the hepatitis B vaccine. HBIG provides immediate passive immunity by delivering pre-formed antibodies, while the vaccine initiates active immunity to prevent future infections (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents). The HBIG should be administered within 24 hours of exposure (preferably within 7 days), and the first dose of the vaccine should be given concurrently, followed by the complete vaccine series.
Option A (immune serum globulin and hepatitis B vaccine) is incorrect because immune serum globulin (ISG) is a general immunoglobulin preparation and not specific for HBV; HBIG, which contains high titers of anti-HBs, is the appropriate specific immunoglobulin for HBV exposure. Option B (hepatitis B immune globulin [HBIG] alone) is insufficient, as it provides only temporary passive immunity without initiating long-term active immunity through vaccination, which is critical for an unvaccinated individual. Option C (hepatitis B vaccine alone) is inadequate for immediate post-exposure protection, as it takes weeks to develop immunity, leaving the employee vulnerable in the interim.
The recommendation for HBIG and hepatitis B vaccine aligns with CBIC’s emphasis on evidence-based post-exposure management to prevent HBV transmission in healthcare settings (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.1 - Collaborate with organizational leaders). This dual approach is supported by CDC guidelines, which prioritize rapid intervention to reduce the risk of seroconversion following percutaneous exposure (CDC Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV, 2013).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.1 - Collaborate with organizational leaders, 3.2 - Implement measures to prevent transmission of infectious agents. CDC Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV, 2013.
Each item or package that is prepared for sterilization should be labeled with the
storage location.
type of sterilization process.
sterilizer identification number or code.
cleaning method (e.g., mechanical or manual).
Answer:
Explanation:
The correct answer is C, "sterilizer identification number or code," as this is the essential information that each item or package prepared for sterilization should be labeled with. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, proper labeling of sterilized items is a critical component of infection prevention and control to ensure traceability and verify the sterilization process. The sterilizer identification number or code links the item to a specific sterilization cycle, allowing the infection preventionist (IP) and sterile processing staff to track the equipment used, confirm compliance with standards (e.g., AAMI ST79), and facilitate recall or investigation if issues arise (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This labeling ensures that the sterility of the item can be assured and documented, protecting patient safety by preventing the use of inadequately processed items.
Option A (storage location) is important for inventory management but is not directly related to the sterilization process itself and does not provide evidence of the sterilization event. Option B (type of sterilization process) indicates the method (e.g., steam, ethylene oxide), which is useful but less critical than the sterilizer identification, as the process type alone does not confirm the specific cycle or equipment used. Option D (cleaning method, e.g., mechanical or manual) is a preliminary step in reprocessing, but it is not required on the sterilization label, as the focus shifts to sterilization verification once the item is prepared.
The requirement for a sterilizer identification number or code aligns with CBIC’s emphasis on maintaining rigorous tracking and quality assurance in the reprocessing of medical devices, ensuring accountability and adherence to best practices (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This practice is mandated by standards such as AAMI ST79 to support effective infection control in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
The Environmental Services department is purchasing a new disinfectant that is an approved hospital disinfectant with no tuberculocidal claim. This product is appropriate for cleaning which of the following items?
Laryngoscope blades
Blood pressure cuff
Respiratory therapy equipment
Ultrasound probe
Answer:
Which performance improvement model should the infection preventionist use to aid in the evaluation of the infection control plan?
Six Sigma
Failure mode and effects analysis
Plan, Do, Study, Act
Root Cause Analysis
Answer:
Explanation:
The Plan, Do, Study, Act (PDSA) model is a widely used performance improvement tool in infection prevention. It focuses on continuous quality improvement through planning, implementing, analyzing data, and making adjustments. This model aligns with infection control program evaluations and The Joint Commission’s infection prevention and control standards.
Why the Other Options Are Incorrect?
A. Six Sigma – A data-driven process improvement method but not as commonly used in infection control as PDSA.
B. Failure Mode and Effects Analysis (FMEA) – Used to identify risks before implementation, rather than ongoing evaluation.
D. Root Cause Analysis (RCA) – Used to analyze failures after they occur, rather than guiding continuous improvement.
CBIC Infection Control Reference
The PDSA cycle is a recognized model for evaluating and improving infection control plans​.
To understand how their hospital-acquired infection rates compare to other health care settings, an infection preventionist (IP) plans to use benchmarking.
Which of the following criteria is important to ensure accurate benchmarking of surveillance data?
Data collectors are trained on how to collect data
Collecting data on a small population lo ensure accuracy of data collection
Denominator rates are selected based on an organizational risk assessment
Using case definitions that are adjusted for the patient population being studied
Answer:
Explanation:
Benchmarking compares infection rates across healthcare facilities. For accurate benchmarking, case definitions must be standardized and adjusted for patient demographics, severity of illness, and other risk factors.
Why the Other Options Are Incorrect?
A. Data collectors are trained on how to collect data – Training is necessary, but it does not directly ensure comparability between facilities.
B. Collecting data on a small population – A larger sample size increases accuracy and reliability in benchmarking.
C. Denominator rates selected based on an organizational risk assessment – Risk assessment is important, but standardized case definitions are critical for comparison.
CBIC Infection Control Reference
According to APIC, accurate benchmarking relies on using standardized case definitions that account for differences in patient populations​.
Which of the following patients with human immunodeficiency virus infection requires Airborne precautions?
24-year-old male newly diagnosed with a CD4 count of 70
28-year-old female with Mycobacterium avium in sputum
36-year-old male with cryptococcal meningitis
46-year-old female with a cavitary lesion in upper lobe
Answer:
Explanation:
HIV patients require Airborne Precautions if they have tuberculosis (TB). A cavitary lesion in the upper lobe is highly suggestive of active pulmonary TB, which requires Airborne Precautions due to aerosolized transmission.
Why the Other Options Are Incorrect?
A. 24-year-old male newly diagnosed with a CD4 count of 70 – Low CD4 count alone does not warrant Airborne Precautions unless there is active TB or another airborne pathogen.
B. 28-year-old female with Mycobacterium avium in sputum – Mycobacterium avium complex (MAC) is not airborne, and standard precautions are sufficient.
C. 36-year-old male with cryptococcal meningitis – Cryptococcus neoformans is not transmitted via the airborne route, so Airborne Precautions are unnecessary.
CBIC Infection Control Reference
Patients with HIV and suspected TB require Airborne Precautions until TB is ruled out​.
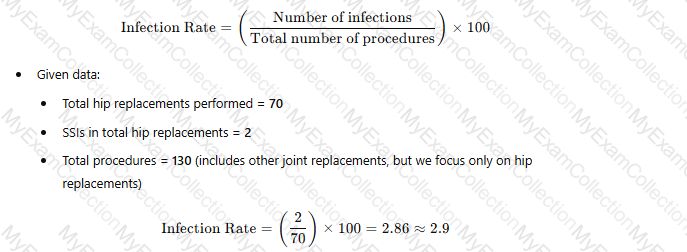
Operating room records indicate that 130 joint replacements have been performed. These include 70 total hip replacements, 55 total knee replacements, and 5 shoulder replacements. Two postoperative surgical site infections (SSIs) were identified in total hip replacements. What is the infection rate/100 procedures for total hip replacements?
1.5
2.9
3.3
3.6
Answer:
Explanation:
To determine the infection rate per 100 procedures for total hip replacements, use the following formula:
 A white paper with black text and numbers
AI-generated content may be incorrect.
A white paper with black text and numbers
AI-generated content may be incorrect.
Thus, the correct answer is B. 2.9 per 100 procedures.
CBIC Infection Control Reference
The methodology of calculating SSI rates aligns with guidelines from the National Healthcare Safety Network (NHSN) and standardized infection ratio (SIR) models used for hospital-specific SSI rates​.
An infection preventionist is evaluating a new catheter that may decrease the rate of catheter-associated urinary tract infections. Which of the following provides the BEST information to support the selection of this catheter?
Staff member preference and product availability
Product materials and vendor information
Value analysis and information provided by the manufacturer
Cost benefit analysis and safety considerations
Answer:
Explanation:
The correct answer is D, "Cost benefit analysis and safety considerations," as this provides the best information to support the selection of a new catheter aimed at decreasing the rate of catheter-associated urinary tract infections (CAUTIs). According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, selecting medical devices like catheters for infection prevention involves a comprehensive evaluation that balances efficacy, safety, and economic impact. A cost-benefit analysis assesses the financial implications (e.g., reduced infection rates leading to lower treatment costs) against the cost of the new catheter, while safety considerations ensure the device minimizes patient risk, such as reducing biofilm formation or irritation that contributes to CAUTIs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This dual focus provides evidence-based data to justify the catheter’s adoption, aligning with the goal of improving patient outcomes and reducing healthcare-associated infections (HAIs).
Option A (staff member preference and product availability) is subjective and logistical rather than evidence-based, making it insufficient for a decision that impacts infection rates. Option B (product materials and vendor information) offers technical details but lacks the broader context of efficacy and cost-effectiveness needed for a comprehensive evaluation. Option C (value analysis and information provided by the manufacturer) includes a structured assessment of value, but it may be biased toward the manufacturer’s claims and lacks the independent safety and cost-benefit perspective critical for infection prevention decisions.
The emphasis on cost-benefit analysis and safety considerations reflects CBIC’s priority on using data-driven and patient-centered approaches to select interventions that enhance infection control (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This approach ensures the catheter’s selection is supported by robust evidence, optimizing both clinical and economic outcomes in the prevention of CAUTIs.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies; Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment.
When developing an exposure control plan, the MOST important aspect in the prevention of exposure to tuberculosis is:
Placement of the patient in an airborne infection isolation room.
Identification of a potentially infectious patient.
Prompt initiation of chemotherapeutic agents.
Use of personal protective equipment.
Answer:
Explanation:
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is an airborne disease that poses a significant risk in healthcare settings, particularly through exposure to infectious droplets. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Prevention and Control of Infectious Diseases" domain, which includes developing exposure control plans, aligning with the Centers for Disease Control and Prevention (CDC) "Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings" (2005). The question seeks the most important aspect of an exposure control plan to prevent TB exposure, requiring a prioritization of preventive strategies.
Option B, "Identification of a potentially infectious patient," is the most important aspect. Early identification of individuals with suspected or confirmed TB (e.g., through symptom screening like persistent cough, fever, or weight loss, or diagnostic tests like chest X-rays and sputum smears) allows for timely isolation and treatment, preventing further transmission. The CDC guidelines stress that the first step in an exposure control plan is to recognize patients with signs or risk factors for infectious TB, as unrecognized cases are the primary source of healthcare worker and patient exposures. The Occupational Safety and Health Administration (OSHA) also mandates risk assessment and early detection as foundational to TB control plans.
Option A, "Placement of the patient in an airborne infection isolation room," is a critical control measure once a potentially infectious patient is identified. Airborne infection isolation rooms (AIIRs) with negative pressure ventilation reduce the spread of infectious droplets, as recommended by the CDC. However, this step depends on prior identification; placing a patient in an AIIR without knowing their infectious status is inefficient and not the initial priority. Option C, "Prompt initiation of chemotherapeutic agents," is essential for treating active TB and reducing infectiousness, typically within days of effective therapy, per CDC guidelines. However, this follows identification and diagnosis (e.g., via acid-fast bacilli smear or culture), making it a secondary action rather than the most important preventive aspect. Option D, "Use of personal protective equipment," such as N95 respirators, is a key protective measure for healthcare workers once an infectious patient is identified, as outlined by the CDC and OSHA. However, PPE is a reactive measure that mitigates exposure after identification and isolation, not the foundational step to prevent it.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize early identification as the cornerstone of TB exposure prevention, enabling all subsequent interventions. Option B ensures that the exposure control plan addresses the source of transmission at its outset, making it the most important aspect.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings, 2005.
OSHA Respiratory Protection Standard, 29 CFR 1910.134.
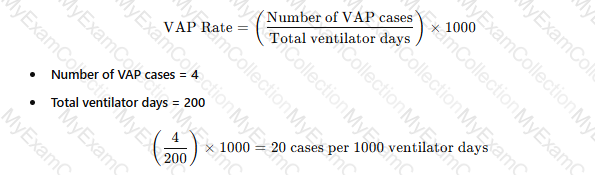
There are four cases of ventilator-associated pneumonia in a surgical intensive care unit with a total of 200 ventilator days and a census of 12 patients. Which of the following BEST expresses how this should be reported?
Ventilator-associated pneumonia rate of 2%
20 ventilator-associated pneumonia cases/1000 ventilator days
Postoperative pneumonia rate of 6% in SICU patients
More information is needed regarding ventilator days per patient
Answer:
Explanation:
The standard way to report ventilator-associated pneumonia (VAP) rates is:
 A white paper with black text
AI-generated content may be incorrect.
A white paper with black text
AI-generated content may be incorrect.
Why the Other Options Are Incorrect?
A. Ventilator-associated pneumonia rate of 2% – This does not use the correct denominator (ventilator days).
C. Postoperative pneumonia rate of 6% in SICU patients – Not relevant, as the data focuses on VAP, not postoperative pneumonia.
D. More information is needed regarding ventilator days per patient – The total ventilator days are already provided, so no additional data is required.
CBIC Infection Control Reference
APIC and NHSN recommend reporting VAP rates as cases per 1,000 ventilator days​.
When implementing a multimodal strategy (or bundle) for improving hand hygiene, the infection preventionist should focus on Calculator
signage for hand hygiene reminders.
cost effectiveness of hand hygiene products.
availability of gloves in the patient care area
institutional assessment of significant barriers.
Answer:
Explanation:
When implementing a multimodal strategy (or bundle) for hand hygiene, the infection preventionist should first assess barriers to compliance before implementing solutions.
Step-by-Step Justification:
Understanding Barriers First:
Identifying barriers (e.g., lack of access to sinks, high workload, or poor compliance culture) is critical for effective intervention​.
APIC Guidelines on Hand Hygiene Improvement:
Strategies must be tailored based on the institution's specific challenges​.
Why Other Options Are Incorrect:
A. Signage for hand hygiene reminders:
Signage alone is insufficient without addressing systemic barriers.
B. Cost-effectiveness of hand hygiene products:
While important, cost analysis comes after identifying compliance barriers.
C. Availability of gloves in the patient care area:
Gloves do not replace hand hygiene and may lead to lower compliance.
CBIC Infection Control References:
APIC/JCR Workbook, "Hand Hygiene Compliance and Institutional Barriers"​.
APIC Text, "Hand Hygiene Improvement Strategies"​.
Essential knowledge, behaviors, and skills that an individual should possess and demonstrate to practice in a specific discipline defines which of the following?
Certification
Competence
Knowledge
Training
Answer:
Explanation:
The correct answer is B, "Competence," as it defines the essential knowledge, behaviors, and skills that an individual should possess and demonstrate to practice in a specific discipline. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, competence encompasses the integrated application of knowledge, skills, and behaviors required to perform effectively in a professional role, such as infection prevention and control. Competence goes beyond mere knowledge or training by including the ability to apply these attributes in real-world scenarios, ensuring safe and effective practice (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.3 - Assess competence of healthcare personnel). This holistic definition is critical in healthcare settings, where demonstrated competence—through actions like proper hand hygiene or outbreak management—directly impacts patient safety and infection prevention outcomes.
Option A (certification) refers to a formal recognition or credential (e.g., CIC certification) that validates an individual’s qualifications, but it is an outcome or process rather than the definition of the underlying abilities. Option C (knowledge) represents the theoretical understanding or factual basis of a discipline, which is a component of competence but not the full scope that includes behaviors and skills. Option D (training) involves the education or instruction provided to develop skills and knowledge, serving as a means to achieve competence rather than defining it.
The focus on competence aligns with CBIC’s emphasis on ensuring that healthcare personnel are equipped to meet the demands of infection prevention through a combination of education, practice, and evaluation (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This definition supports the development of professionals who can adapt and perform effectively in dynamic healthcare environments.
References: CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competencies 4.2 - Evaluate the effectiveness of educational programs, 4.3 - Assess competence of healthcare personnel.
A 21-ycnr-old college student was admitted with a high fever. The Emergency Department physician be gan immediate treatment with intravenous vancomycin and ceftriaxone while awaiting blood, urine, and cerebrospinal fluid cultures. The following day. the cultures of both the blood and the cerebrospinal fluid were reported to be growing meningococci. The patient was placed on precautions on admission. Which of the following is correct?
Droplet precautions may be discontinued after 24 hours of therapy.
Droplet precautions must continue
Airborne precautions may be discontinued after 24 hours of therapy.
Airborne precautions must continue.
Answer:
Explanation:
Meningococcal infections, such as Neisseria meningitidis, are transmitted via respiratory droplets. According to APIC and CDC guidelines, patients with meningococcal disease should be placed on Droplet Precautions upon admission. These precautions can be discontinued after 24 hours of effective antibiotic therapy.
Why the Other Options Are Incorrect?
B. Droplet precautions must continue – Droplet Precautions are not needed beyond 24 hours of appropriate therapy because treatment rapidly reduces infectiousness.
C. Airborne precautions may be discontinued after 24 hours of therapy – Meningococcal infection is not airborne, so Airborne Precautions are never required.
D. Airborne precautions must continue – Incorrect because meningococci do not transmit via airborne particles.
CBIC Infection Control Reference
According to APIC guidelines, Droplet Precautions should be maintained for at least 24 hours after effective antibiotic therapy initiation​.
Healthcare workers are MOST likely to benefit from infection prevention education if the Infection Preventionist (IP)
brings in speakers who are recognized experts.
plans the educational program well ahead of time.
audits practices and identifies deficiencies.
involves the staff in determining the content.
Answer:
Explanation:
The correct answer is D, "involves the staff in determining the content," as this approach is most likely to benefit healthcare workers from infection prevention education. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective education programs are tailored to the specific needs and contexts of the learners. Involving staff in determining the content ensures that the educational material addresses their real-world challenges, knowledge gaps, and interests, thereby increasing engagement, relevance, and application of the learned principles (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). This participatory approach fosters ownership and accountability among healthcare workers, enhancing the likelihood that they will adopt and sustain infection prevention practices.
Option A (brings in speakers who are recognized experts) can enhance credibility and provide high-quality information, but it does not guarantee that the content will meet the specific needs of the staff unless their input is considered. Option B (plans the educational program well ahead of time) is important for logistical success and preparedness, but without staff involvement, the program may lack relevance or fail to address immediate concerns. Option C (audits practices and identifies deficiencies) is a valuable step in identifying areas for improvement, but it is a diagnostic process rather than a direct educational strategy; education based solely on audits might not engage staff effectively if their input is not sought.
The focus on involving staff aligns with CBIC’s emphasis on adult learning principles, which highlight the importance of learner-centered education. By involving staff, the IP adheres to best practices for adult education, ensuring that the program is practical and tailored, ultimately leading to better outcomes in infection prevention (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This approach also supports a collaborative culture, which is critical for sustaining infection control efforts in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competencies 4.1 - Develop and implement educational programs, 4.2 - Evaluate the effectiveness of educational programs.
Which of the following BEST demonstrates the effectiveness of a program targeted at reducing central-line associated bloodstream infections (CLABSIs) in an intensive care unit (ICU)?
A 25% decrease in the length of stay in the ICU related to CLABSIs
A 25% reduction in the incidence of CLABSIs over 6 months
A 30% decrease in total costs related to treatment of CLABSIs over 12 months
A 30% reduction in the use of antibiotic-impregnated central catheters over 6 months
Answer:
Explanation:
Evaluating the effectiveness of a program to reduce central-line associated bloodstream infections (CLABSIs) in an intensive care unit (ICU) requires identifying the most direct and relevant measure of success. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes outcome-based assessment in the "Performance Improvement" and "Surveillance and Epidemiologic Investigation" domains, aligning with the Centers for Disease Control and Prevention (CDC) guidelines for infection prevention. The primary goal of a CLABSI reduction program is to decrease the occurrence of these infections, with secondary benefits including reduced length of stay, costs, and resource use.
Option B, "A 25% reduction in the incidence of CLABSIs over 6 months," is the best demonstration of effectiveness. The incidence of CLABSIs—defined by the CDC as the number of infections per 1,000 central line days—directly measures the program’s impact on the targeted outcome: preventing bloodstream infections associated with central lines. A 25% reduction over 6 months indicates a sustained decrease in infection rates, providing clear evidence that the intervention (e.g., improved insertion techniques, maintenance bundles, or staff education) is working. The CDC’s "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) and the National Healthcare Safety Network (NHSN) protocols prioritize infection rate reduction as the primary metric for assessing CLABSI prevention programs.
Option A, "A 25% decrease in the length of stay in the ICU related to CLABSIs," is a secondary benefit. Reducing CLABSI-related length of stay can improve patient outcomes and bed availability, but it is an indirect measure dependent on infection incidence. A decrease in length of stay could also reflect other factors (e.g., improved discharge planning), making it less specific to program effectiveness. Option C, "A 30% decrease in total costs related to treatment of CLABSIs over 12 months," reflects a financial outcome, which is valuable for justifying resource allocation. However, cost reduction is a downstream effect of decreased infections and may be influenced by variables like hospital pricing or treatment protocols, diluting its direct link to program success. Option D, "A 30% reduction in the use of antibiotic-impregnated central catheters over 6 months," indicates a change in practice but not necessarily effectiveness. Antibiotic-impregnated catheters are one prevention strategy, and reducing their use could suggest improved standard practices (e.g., chlorhexidine bathing), but it could also increase infection rates if not offset by other measures, making it an ambiguous indicator.
The CBIC Practice Analysis (2022) and CDC guidelines emphasize that the primary measure of a CLABSI prevention program’s success is a reduction in infection incidence, as it directly addresses patient safety and the program’s core objective. Option B provides the most robust and specific evidence of effectiveness over a defined timeframe.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2017.
NHSN CLABSI Surveillance Protocol, 2021.
Which of the following is the correct collection technique to obtain a laboratory specimen for suspected pertussis?
Cough plate
Nares culture
Sputum culture
Nasopharyngeal culture
Answer:
Explanation:
The gold standard specimen for diagnosing pertussis (Bordetella pertussis infection) is a nasopharyngeal culture because:
B. pertussis colonizes the nasopharynx, making it the best site for detection.
A properly collected nasopharyngeal swab or aspirate increases diagnostic sensitivity.
This method is recommended for culture, PCR, or direct fluorescent antibody testing.
Why the Other Options Are Incorrect?
A. Cough plate – Not commonly used due to low sensitivity.
B. Nares culture – The nares are not a primary site for pertussis colonization.
C. Sputum culture – B. pertussis does not commonly infect the lower respiratory tract.
CBIC Infection Control Reference
APIC confirms that nasopharyngeal culture is the preferred method for diagnosing pertussis​.
Peripherally inserted central catheter (PICC)-associated bloodstream infections (BSIs) have been increasing over the past four months. Which of the following interventions is MOST likely to have contributed to the increase?
Use of chlorhexidine skin antisepsis during insertion of the PICC
Daily bathing adult intensive care unit patients with chlorhexidine
Replacement of the intravenous administration sets every 72 hours
Use of a positive pressure device on the PICC
Answer:
Explanation:
Peripherally inserted central catheter (PICC)-associated bloodstream infections (BSIs) are a significant concern in healthcare settings, and identifying factors contributing to their increase is critical for infection prevention. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Surveillance and Epidemiologic Investigation" and "Prevention and Control of Infectious Diseases" domains, which align with the Centers for Disease Control and Prevention (CDC) guidelines for preventing intravascular catheter-related infections. The question asks for the intervention most likely to have contributed to the rise in PICC-associated BSIs over four months, requiring an evaluation of each option based on evidence-based practices.
Option C, "Replacement of the intravenous administration sets every 72 hours," is the most likely contributor to the increase. The CDC’s "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) recommend that intravenous administration sets (e.g., tubing for fluids or medications) be replaced no more frequently than every 72-96 hours unless clinically indicated (e.g., contamination or specific therapy requirements). Frequent replacement, such as every 72 hours as a routine practice, can introduce opportunities for contamination during the change process, especially if aseptic technique is not strictly followed. Studies cited in the CDC guidelines, including those by O’Grady et al. (2011), indicate that unnecessary manipulation of catheter systems increases the risk of introducing pathogens, potentially leading to BSIs. A change to a 72-hour replacement schedule, if not previously standard, could explain the observed increase over the past four months.
Option A, "Use of chlorhexidine skin antisepsis during insertion of the PICC," is a recommended practice to reduce BSIs. Chlorhexidine, particularly in a 2% chlorhexidine gluconate with 70% alcohol solution, is the preferred skin antiseptic for catheter insertion due to its broad-spectrum activity and residual effect, as supported by the CDC (2017). This intervention should decrease, not increase, infection rates, making it an unlikely contributor. Option B, "Daily bathing adult intensive care unit patients with chlorhexidine," is another evidence-based strategy to reduce healthcare-associated infections, including BSIs, by decolonizing the skin of pathogens like Staphylococcus aureus. The CDC and SHEA (Society for Healthcare Epidemiology of America) guidelines (2014) endorse chlorhexidine bathing in intensive care units, suggesting it should lower, not raise, BSI rates. Option D, "Use of a positive pressure device on the PICC," aims to prevent catheter occlusion and reduce the need for frequent flushing, which could theoretically decrease infection risk by minimizing manipulation. However, there is no strong evidence linking positive pressure devices to increased BSIs; if improperly used or maintained, they might contribute marginally, but this is less likely than the impact of frequent tubing changes.
The CBIC Practice Analysis (2022) and CDC guidelines highlight that deviations from optimal catheter maintenance practices, such as overly frequent administration set replacements, can increase infection risk. Given the four-month timeframe and the focus on an intervention’s potential negative impact, Option C stands out as the most plausible contributor due to the increased manipulation and contamination risk associated with routine 72-hour replacements.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2017.
O’Grady, N. P., et al. (2011). Guidelines for the Prevention of Intravascular Catheter-Related Infections. Clinical Infectious Diseases.
SHEA Compendium, Strategies to Prevent Central Line-Associated Bloodstream Infections, 2014.
Which statistical test is MOST appropriate for comparing infection rates before and after an intervention?
Student’s t-test
Chi-square test for proportions
Linear regression analysis
Wilcoxon rank-sum test
Answer:
Explanation:
The Chi-square test is the most appropriate test for comparing infection rates (categorical data) before and after an intervention​.
CBIC Infection Control References:
CIC Study Guide, "Statistical Analysis in Infection Control," Chapter 5​.
The cleaning and disinfection process that is appropriate for a particular surgical instrument depends on
all surgical instruments are cleaned and sterilized in the same manner.
instruments contaminated with blood must be bleach cleaned first.
the device manufacturer's written instructions for use.
the policies of the sterile processing department.
Answer:
Explanation:
The correct answer is C, "the device manufacturer's written instructions for use," as this is the factor that determines the appropriate cleaning and disinfection process for a particular surgical instrument. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the reprocessing of surgical instruments must follow the specific instructions provided by the device manufacturer to ensure safety and efficacy. These instructions account for the instrument’s material, design, and intended use, specifying the appropriate cleaning agents, disinfection methods, sterilization techniques, and contact times to prevent damage and ensure the elimination of pathogens (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This is also mandated by regulatory standards, such as those from the Food and Drug Administration (FDA) and the Association for the Advancement of Medical Instrumentation (AAMI), which require adherence to manufacturer guidelines to maintain device integrity and patient safety.
Option A (all surgical instruments are cleaned and sterilized in the same manner) is incorrect because different instruments have unique characteristics (e.g., materials like stainless steel vs. delicate optics), necessitating tailored reprocessing methods rather than a one-size-fits-all approach. Option B (instruments contaminated with blood must be bleach cleaned first) is a misconception; while blood contamination requires thorough cleaning, bleach is not universally appropriate and may damage certain instruments unless specified by the manufacturer. Option D (the policies of the sterile processing department) may guide internal procedures but must be based on and subordinate to the manufacturer’s instructions to ensure compliance and effectiveness.
The emphasis on manufacturer instructions aligns with CBIC’s focus on evidence-based reprocessing practices to prevent healthcare-associated infections (HAIs) and protect patients (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). Deviating from these guidelines can lead to inadequate sterilization or instrument damage, increasing infection risks.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
An infection preventionist in the role of educator is teaching risk reduction activities to patients and families. For which of the following groups is the pneumococcal vaccine MOST appropriate?
Asplenic patients
International travelers
Immunocompromised newborns
Patients in behavioral health settings
Answer:
Explanation:
The pneumococcal vaccine is designed to protect against infections caused by Streptococcus pneumoniae, a bacterium responsible for diseases such as pneumonia, meningitis, and bacteremia. The appropriateness of this vaccine depends on the population's risk profile, particularly their susceptibility to invasive pneumococcal disease (IPD). The Certification Board of Infection Control and Epidemiology (CBIC) highlights the role of infection preventionists as educators in promoting vaccination as a key risk reduction strategy, aligning with the "Education and Training" domain (CBIC Practice Analysis, 2022). The Centers for Disease Control and Prevention (CDC) provides specific guidelines on pneumococcal vaccination, recommending it for individuals at higher risk due to underlying medical conditions or immunologic status.
Option A, asplenic patients, refers to individuals who have had their spleen removed (e.g., due to trauma or disease) or have a nonfunctional spleen (e.g., in sickle cell disease). The spleen plays a critical role in clearing encapsulated bacteria like Streptococcus pneumoniae from the bloodstream. Without a functioning spleen, these patients are at significantly increased risk of overwhelming post-splenectomy infection (OPSI), with pneumococcal disease being a leading cause. The CDC and Advisory Committee on Immunization Practices (ACIP) strongly recommend pneumococcal vaccination, including both PCV15/PCV20 and PPSV23, for asplenic patients, making this group the most appropriate for the vaccine in this context. The infection preventionist should prioritize educating these patients and their families about the vaccine's importance and timing.
Option B, international travelers, may benefit from various vaccines depending on their destination (e.g., yellow fever or typhoid), but pneumococcal vaccination is not routinely recommended unless they have specific risk factors (e.g., asplenia or chronic illness) or are traveling to areas with high pneumococcal disease prevalence. This group is not inherently a priority for pneumococcal vaccination. Option C, immunocompromised newborns, includes infants with congenital immunodeficiencies or other conditions, who may indeed require pneumococcal vaccination as part of their routine immunization schedule (e.g., PCV15 or PCV20 starting at 2 months). However, newborns are generally covered under universal childhood vaccination programs, and the question’s focus on "MOST appropriate" suggests a group with a more specific, elevated risk, which asplenic patients fulfill. Option D, patients in behavioral health settings, may have varied health statuses, but this group is not specifically targeted for pneumococcal vaccination unless they have additional risk factors (e.g., chronic diseases), making it less appropriate than asplenic patients.
The CBIC emphasizes tailoring education to high-risk populations, and the CDC’s Adult and Pediatric Immunization Schedules (2023) identify asplenic individuals as a top priority for pneumococcal vaccination due to their extreme vulnerability. Thus, the infection preventionist should focus on asplenic patients as the group for whom the pneumococcal vaccine is most appropriate.
References:
CBIC Practice Analysis, 2022.
CDC Adult Immunization Schedule, 2023.
CDC Pediatric Immunization Schedule, 2023.
ACIP Recommendations for Pneumococcal Vaccination, 2022.
An 84-year-old male with a gangrenous foot is admitted to the hospital from an extended-care facility (ECF). The ECF is notified that the wound grew Enterococcus faecium with the following antibiotic sensitivity results:
ampicillin – R
vancomycin – R
penicillin – R
linezolid – S
This is the fourth Enterococcus species cultured from residents within the same ECF wing in the past month. The other cultures were from two urine specimens and a draining wound. The Infection Preventionist (IP) should immediately:
Notify the medical director of the outbreak.
Compare the four culture reports and sensitivity patterns.
Conduct surveillance cultures for this organism in all residents.
Notify the nursing administrator to close the wing to new admissions.
Answer:
Explanation:
The scenario describes a potential outbreak of multidrug-resistant Enterococcus faecium in an extended-care facility (ECF) wing, indicated by four positive cultures (including the current case and three prior cases from urine and a draining wound) within a month. The organism exhibits resistance to ampicillin, vancomycin, and penicillin, but sensitivity to linezolid, suggesting a possible vancomycin-resistant Enterococcus (VRE) strain, which is a significant concern in healthcare settings. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of rapid outbreak detection and response in the "Surveillance and Epidemiologic Investigation" domain, aligning with Centers for Disease Control and Prevention (CDC) guidelines for managing multidrug-resistant organisms (MDROs).
Option A, "Notify the medical director of the outbreak," is the most immediate and critical action. Identifying an outbreak—defined by the CDC as two or more cases of a similar illness linked by time and place—requires prompt notification to the facility’s leadership (e.g., medical director) to initiate a coordinated response. The presence of four Enterococcus cases, including a multidrug-resistant strain, within a single ECF wing over a month suggests a potential cluster, necessitating urgent action to assess the scope, implement control measures, and allocate resources. The CDC’s "Management of Multidrug-Resistant Organisms in Healthcare Settings" (2006) recommends immediate reporting to facility leadership as the first step to activate an outbreak investigation team, making this the priority.
Option B, "Compare the four culture reports and sensitivity patterns," is an important subsequent step in outbreak investigation. Analyzing the antibiotic susceptibility profiles and culture sources can confirm whether the cases are epidemiologically linked (e.g., clonal spread of VRE) and guide treatment and control strategies. However, this is a detailed analysis that follows initial notification and should not delay alerting the medical director. Option C, "Conduct surveillance cultures for this organism in all residents," is a proactive measure to determine the prevalence of Enterococcus faecium, especially VRE, within the wing. The CDC recommends targeted surveillance during outbreaks, but this requires prior authorization and planning by the outbreak team, making it a secondary action after notification. Option D, "Notify the nursing administrator to close the wing to new admissions," may be a control measure to prevent further spread, as suggested by the CDC for MDRO outbreaks. However, closing a unit is a significant decision that should be guided by the medical director and infection control team after assessing the situation, not an immediate independent action by the IP.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize rapid communication with leadership to initiate a structured outbreak response, including resource allocation and policy adjustments. Given the multidrug-resistant nature and cluster pattern, notifying the medical director (Option A) is the most immediate and appropriate action to ensure a comprehensive response.
References:
CBIC Practice Analysis, 2022.
CDC Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006.
Hand hygiene rates in the facility have been decreasing over time. The Infection Preventionist (IP) surveys staff and finds that hand dryness is the major reason for non-compliance. What step should the IP take?
Provide staff lotion in every patient room.
Provide a compatible lotion in a convenient location.
Allow staff to bring in lotion and carry it in their pockets.
Allow staff to bring in lotion for use at the nurses’ station and lounge.
Answer:
Explanation:
Hand hygiene is a cornerstone of infection prevention, and declining compliance rates pose a significant risk for healthcare-associated infections (HAIs). The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes improving hand hygiene adherence in the "Prevention and Control of Infectious Diseases" domain, aligning with the Centers for Disease Control and Prevention (CDC) "Guideline for Hand Hygiene in Healthcare Settings" (2002). The IP’s survey identifies hand dryness as the primary barrier, likely due to the frequent use of alcohol-based hand sanitizers or soap, which can dehydrate skin. The goal is to address this barrier effectively while maintaining infection control standards.
Option B, "Provide a compatible lotion in a convenient location," is the most appropriate step. The CDC and World Health Organization (WHO) recommend using moisturizers to mitigate skin irritation and dryness, which can improve hand hygiene compliance. However, the lotion must be compatible with alcohol-based hand rubs (e.g., free of petroleum-based products that can reduce sanitizer efficacy) and placed in accessible areas (e.g., near sinks or sanitizer dispensers) to encourage use without disrupting workflow. The WHO’s "Guidelines on Hand Hygiene in Health Care" (2009) suggest providing skin care products as part of a multimodal strategy to enhance adherence, making this a proactive, facility-supported solution that addresses the root cause.
Option A, "Provide staff lotion in every patient room," is a good intention but impractical and potentially risky. Placing lotion in patient rooms could lead to inconsistent use, contamination (e.g., from patient contact), or misuse (e.g., staff applying incompatible products), compromising infection control. The CDC advises against uncontrolled lotion distribution in patient care areas. Option C, "Allow staff to bring in lotion and carry it in their pockets," introduces variability in product quality and compatibility. Personal lotions may contain ingredients (e.g., oils) that inactivate alcohol-based sanitizers, and pocket storage increases the risk of contamination or cross-contamination, which the CDC cautions against. Option D, "Allow staff to bring in lotion for use at the nurses’ station and lounge," limits the intervention to non-patient care areas, reducing its impact on hand hygiene during patient interactions. It also shares the compatibility and contamination risks of Option C, making it less effective.
The CBIC Practice Analysis (2022) and CDC guidelines emphasize evidence-based interventions, such as providing approved skin care products in strategic locations to boost compliance. Option B balances accessibility, safety, and compatibility, making it the best step to address hand dryness and improve hand hygiene rates.
References:
CBIC Practice Analysis, 2022.
CDC Guideline for Hand Hygiene in Healthcare Settings, 2002.
WHO Guidelines on Hand Hygiene in Health Care, 2009.
A surgeon is beginning a new procedure in the facility within the next two weeks and requires loaner instruments. Infection prevention processes should ensure that
items arrive in time for immediate use steam sterilization.
instruments are able to be used prior to the biological indicator results.
the planning process takes place after the instruments have arrived.
staff education related to loaner instrument reprocessing has occurred.
Answer:
Explanation:
The correct answer is D, "staff education related to loaner instrument reprocessing has occurred," as this is the infection prevention process that should be ensured when a surgeon is beginning a new procedure requiring loaner instruments within the next two weeks. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, loaner instruments—those borrowed from external sources for temporary use—pose unique infection prevention challenges due to potential variability in reprocessing standards and unfamiliarity among staff. Ensuring that staff are educated on proper reprocessing protocols (e.g., cleaning, sterilization, and handling per manufacturer instructions and AAMI ST79) is critical to prevent healthcare-associated infections (HAIs) (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This education should cover the specific requirements for loaner instruments, including documentation and verification of sterilization, and should occur proactively before the instruments are used to ensure competency and compliance.
Option A (items arrive in time for immediate use steam sterilization) is a logistical consideration, but it does not address the infection prevention process itself; timely arrival is necessary but insufficient without proper reprocessing validation. Option B (instruments are able to be used prior to the biological indicator results) is unsafe, as biological indicators are essential to confirm sterilization efficacy, and using instruments before results are available violates infection control standards. Option C (the planning process takes place after the instruments have arrived) is impractical, as planning (e.g., coordinating with vendors, assessing reprocessing needs) must occur in advance to ensure readiness and safety, not as a reactive step.
The focus on staff education aligns with CBIC’s emphasis on preparing healthcare personnel to handle loaner instruments safely, reducing the risk of contamination and ensuring patient safety (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). This proactive measure is supported by AAMI and CDC guidelines, which stress the importance of training for reprocessing complex or unfamiliar devices.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment; Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
When evaluating environmental cleaning and disinfectant products as a part of the product evaluation committee, which of the following is responsible for providing information regarding clinical trials?
Infection Preventionist
Clinical representatives
Environmental Services
Manufacturer representatives
Answer:
Explanation:
The correct answer is D, "Manufacturer representatives," as they are responsible for providing information regarding clinical trials when evaluating environmental cleaning and disinfectant products as part of the product evaluation committee. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, manufacturers are the primary source of data on the efficacy, safety, and performance of their products, including clinical trial results that demonstrate the disinfectant’s ability to reduce microbial load or prevent healthcare-associated infections (HAIs) (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). This information is critical for the committee to assess whether the product meets regulatory standards (e.g., EPA registration) and aligns with infection prevention goals, and it is typically supported by documentation such as peer-reviewed studies or trial data provided by the manufacturer.
Option A (Infection Preventionist) plays a key role in evaluating the product’s fit within infection control practices and may contribute expertise or conduct internal assessments, but they are not responsible for providing clinical trial data, which originates from the manufacturer. Option B (Clinical representatives) can offer insights into clinical usage and outcomes but rely on manufacturer data for trial evidence rather than generating it. Option C (Environmental Services) focuses on the practical application and cleaning processes but lacks the authority or resources to conduct or provide clinical trial information.
The reliance on manufacturer representatives aligns with CBIC’s emphasis on evidence-based decision-making in product selection, ensuring that the product evaluation committee bases its choices on robust, manufacturer-supplied clinical data (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This approach supports the safe and effective implementation of environmental cleaning products in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies; Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols.
Which of the following individuals should be excluded from receiving live attenuated influenza virus?
Pregnant persons
Healthy persons aged 2 to 49
Persons with allergies to chicken feathers
Persons simultaneously receiving an inactivated vaccine
Answer:
Explanation:
The correct answer is A, "Pregnant persons," as they should be excluded from receiving the live attenuated influenza virus (LAIV) vaccine. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, which align with recommendations from the Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP), the LAIV, commonly known as the nasal spray flu vaccine, contains a live attenuated form of the influenza virus. This vaccine is contraindicated in pregnant individuals due to the theoretical risk of the attenuated virus replicating and potentially harming the fetus, despite limited evidence of adverse outcomes (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents). Pregnant persons are instead recommended to receive the inactivated influenza vaccine (IIV), which is considered safe during pregnancy.
Option B (healthy persons aged 2 to 49) is incorrect because this group is generally eligible to receive LAIV, provided they have no other contraindications, as the vaccine is approved for healthy, non-pregnant individuals in this age range (CDC Immunization Schedules, 2024). Option C (persons with allergies to chicken feathers) is not a contraindication for LAIV; the vaccine is produced in eggs, and while egg allergy was historically a concern, current guidelines indicate that LAIV can be administered to persons with egg allergies if they can tolerate egg in their diet, with precautions managed by healthcare providers. Option D (persons simultaneously receiving an inactivated vaccine) is also incorrect, as LAIV can be co-administered with inactivated vaccines without issue, according to ACIP recommendations, as there is no significant interference between the two vaccine types.
The exclusion of pregnant persons reflects CBIC’s emphasis on tailoring infection prevention strategies, including vaccination programs, to protect vulnerable populations while minimizing risks (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.1 - Collaborate with organizational leaders). This decision is based on precautionary principles outlined in CDC and ACIP guidelines to ensure maternal and fetal safety (CDC Prevention and Control of Seasonal Influenza with Vaccines, 2023).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.1 - Collaborate with organizational leaders, 3.2 - Implement measures to prevent transmission of infectious agents. CDC Prevention and Control of Seasonal Influenza with Vaccines, 2023. CDC Immunization Schedules, 2024.
An infection preventionist is reviewing a wound culture result on a surgery patient. The abdominal wound culture of purulent drainage grew Staphylococcus aureus with the following sensitivity pattern: resistant to penicillin, oxacillin, cephalothin, and erythromycin; susceptible to clindamycin, and vancomycin. The patient is currently being treated with cefazolin. Which of the following is true?
The wound is not infected.
The current therapy is not effective.
Droplet Precautions should be initiated.
This is a methicillin-sensitive S. aureus (MSSA) strain.
Answer:
Explanation:
The scenario involves a surgical patient with a purulent abdominal wound culture growing Staphylococcus aureus, a common pathogen in surgical site infections (SSIs). The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes accurate interpretation of culture results and antibiotic therapy in the "Identification of Infectious Disease Processes" and "Prevention and Control of Infectious Diseases" domains, aligning with the Centers for Disease Control and Prevention (CDC) guidelines for managing SSIs. The question requires assessing the sensitivity pattern and current treatment to determine the correct statement.
Option B, "The current therapy is not effective," is true. The wound culture shows Staphylococcus aureus resistant to oxacillin, indicating methicillin-resistant S. aureus (MRSA). The sensitivity pattern lists resistance to penicillin, oxacillin, cephalothin, and erythromycin, with susceptibility to clindamycin and vancomycin. Cefazolin, a first-generation cephalosporin, is ineffective against MRSA because resistance to oxacillin (a penicillinase-resistant penicillin) implies cross-resistance to cephalosporins like cefazolin due to altered penicillin-binding proteins (PBPs). The CDC’s "Guidelines for the Prevention of Surgical Site Infections" (2017) and the Clinical and Laboratory Standards Institute (CLSI) standards confirm that MRSA strains are not susceptible to cefazolin, meaning the current therapy is inappropriate and unlikely to resolve the infection, supporting Option B.
Option A, "The wound is not infected," is incorrect. The presence of purulent drainage, a clinical sign of infection, combined with a positive culture for S. aureus, confirms an active wound infection. The CBIC and CDC define purulent discharge as a key indicator of SSI, ruling out this statement. Option C, "Droplet Precautions should be initiated," is not applicable. Droplet Precautions are recommended for pathogens transmitted via respiratory droplets (e.g., influenza, pertussis), not for S. aureus, which is primarily spread by contact. The CDC’s "Guideline for Isolation Precautions" (2007) specifies Contact Precautions for MRSA, not Droplet Precautions, making this false. Option D, "This is a methicillin-sensitive S. aureus (MSSA) strain," is incorrect. Methicillin sensitivity is determined by susceptibility to oxacillin, and the resistance to oxacillin in the culture result classifies this as MRSA, not MSSA. The CDC and CLSI use oxacillin resistance as the defining criterion for MRSA.
The CBIC Practice Analysis (2022) and CDC guidelines stress the importance of aligning antimicrobial therapy with sensitivity patterns to optimize treatment outcomes. The mismatch between cefazolin and the MRSA sensitivity profile confirms that Option B is the correct statement, indicating ineffective current therapy.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for the Prevention of Surgical Site Infections, 2017.
CDC Guideline for Isolation Precautions, 2007.
CLSI Performance Standards for Antimicrobial Susceptibility Testing, 2022.
Which of the following intravenous solutions will MOST likely promote the growth of microorganisms?
50% hypertonic glucose
5% dextrose
Synthetic amino acids
10% lipid emulsions
Answer:
Explanation:
10% lipid emulsions are the most likely to promote microbial growth because they provide an ideal environment for bacterial and fungal proliferation, especially Staphylococcus aureus, Pseudomonas aeruginosa, and Candida species. Lipids support rapid bacterial multiplication due to their high nutrient content.
Why the Other Options Are Incorrect?
A. 50% hypertonic glucose – High glucose concentrations inhibit bacterial growth due to osmotic pressure effects.
B. 5% dextrose – While it can support some bacterial growth, it is less favorable than lipid emulsions.
C. Synthetic amino acids – These solutions do not support microbial growth as well as lipid emulsions.
CBIC Infection Control Reference
APIC guidelines confirm that lipid-based solutions support rapid microbial growth and should be handled with strict aseptic technique​.
A patient has an oral temperature of 101° F (38.33 C). Erythema and tenderness arc noted at the central line site. Blood samples are submitted for culture and intravenous vancomycin is ordered. This is an example of which of the following forms of antibiotic treatment?
Empiric
Prophylactic
Experimental
Broad spectrum
Answer:
Explanation:
Empiric antibiotic therapy is the immediate initiation of antibiotics based on clinical judgment before laboratory confirmation of an infection. In this case, the presence of fever, erythema, and tenderness at the central line site suggests a possible bloodstream infection, prompting empiric treatment with vancomycin.
Step-by-Step Justification:
Initiation Before Lab Confirmation:
Empiric therapy starts treatment based on symptoms while awaiting culture results​.
Prevents Complications:
Delayed treatment in central line-associated bloodstream infections (CLABSI) can lead to sepsis.
Common in High-Risk Situations:
Empiric treatment is used in cases where waiting for lab results could worsen the patient’s condition.
Why Other Options Are Incorrect:
B. Prophylactic:
Prophylactic antibiotics are given to prevent infection, not to treat an existing one.
C. Experimental:
Experimental treatment refers to clinical trials or unproven therapies, which does not apply here.
D. Broad spectrum:
Broad-spectrum antibiotics cover multiple bacteria, but empiric therapy may be narrow-spectrum based on suspected pathogens​.
CBIC Infection Control References:
APIC Text, Chapter on Antimicrobial Stewardship and Empiric Therapy​.
Following recent renovations on an oncology unit, three patients were identified with Aspergillus infections. The infections were thought to be facility-acquired. Appropriate environmental microbiological monitoring would be to culture the:
Air
Ice
Carpet
Aerators
Answer:
Explanation:
The scenario describes an outbreak of Aspergillus infections among three patients on an oncology unit following recent renovations, with the infections suspected to be facility-acquired. Aspergillus is a mold commonly associated with environmental sources, particularly airborne spores, and its presence in immunocompromised patients (e.g., oncology patients) poses a significant risk. The infection preventionist must identify the appropriate environmental microbiological monitoring strategy, guided by the Certification Board of Infection Control and Epidemiology (CBIC) and CDC recommendations. Let’s evaluate each option:
A. Air: Aspergillus species are ubiquitous molds that thrive in soil, decaying vegetation, and construction dust, and they are primarily transmitted via airborne spores. Renovations can disturb these spores, leading to aerosolization and inhalation by vulnerable patients. Culturing the air using methods such as settle plates, air samplers, or high-efficiency particulate air (HEPA) filtration monitoring is a standard practice to detect Aspergillus during construction or post-renovation in healthcare settings, especially oncology units where patients are at high risk for invasive aspergillosis. This aligns with CBIC’s emphasis on environmental monitoring for airborne pathogens, making it the most appropriate choice.
B. Ice: Ice can be a source of contamination with bacteria (e.g., Pseudomonas, Legionella) or other pathogens if improperly handled or stored, but it is not a typical reservoir for Aspergillus, which is a mold requiring organic material and moisture for growth. While ice safety is important in infection control, culturing ice is irrelevant to an Aspergillus outbreak linked to renovations and is not a priority in this context.
C. Carpet: Carpets can harbor dust, mold, and other microorganisms, especially in high-traffic or poorly maintained areas. Aspergillus spores could theoretically settle in carpet during renovations, but carpets are not a primary source of airborne transmission unless disturbed (e.g., vacuuming). Culturing carpet might be a secondary step if air sampling indicates widespread contamination, but it is less direct and less commonly recommended as the initial monitoring site compared to air sampling.
D. Aerators: Aerators (e.g., faucet aerators) can harbor waterborne pathogens like Pseudomonas or Legionella due to biofilm formation, but Aspergillus is not typically associated with water systems unless there is significant organic contamination or aerosolization from water sources (e.g., cooling towers). Culturing aerators is relevant for waterborne outbreaks, not for an Aspergillus outbreak linked to renovations, making this option inappropriate.
The best answer is A, culturing the air, as Aspergillus is an airborne pathogen, and renovations are a known risk factor for spore dispersal in healthcare settings. This monitoring strategy allows the infection preventionist to confirm the source, assess the extent of contamination, and implement control measures (e.g., enhanced filtration, construction barriers) to protect patients. This is consistent with CBIC and CDC guidelines for managing fungal outbreaks in high-risk units.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which recommends air sampling for Aspergillus during construction-related outbreaks.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes environmental monitoring for facility-acquired infections.
CDC Guidelines for Environmental Infection Control in Healthcare Facilities (2022), which advocate air culturing to detect Aspergillus post-renovation in immunocompromised patient areas.
An infection preventionist is utilizing the Shewhart/Deming cycle in an infection control program performance improvement project. In which of the following steps are the results of the interventions compared with the original goal?
Do
Act
Plan
Study
Answer:
Explanation:
The correct answer is D, "Study," as this is the step in the Shewhart/Deming cycle (commonly known as the Plan-Do-Study-Act [PDSA] cycle) where the results of the interventions are compared with the original goal. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the PDSA cycle is a systematic approach to quality improvement, widely used in infection control programs to test and refine interventions. The cycle consists of four stages: Plan (designing the intervention and setting goals), Do (implementing the intervention on a small scale), Study (analyzing the data and comparing outcomes against the original goal), and Act (standardizing successful changes or adjusting based on findings) (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). The Study phase is critical for assessing whether the intervention achieved the intended reduction in infection rates or other performance metrics, providing evidence to guide the next steps.
Option A (Do) involves the execution of the planned intervention, focusing on implementation rather than evaluation, so it does not include comparing results. Option B (Act) is the final step where successful interventions are implemented on a broader scale or adjustments are made, but it follows the comparison made in the Study phase. Option C (Plan) is the initial stage of setting objectives and designing the intervention, which occurs before any results are available for comparison.
The emphasis on the Study phase aligns with CBIC’s focus on using data to evaluate the effectiveness of infection prevention strategies, ensuring that performance improvement projects are evidence-based and goal-oriented (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions). This step enables the infection preventionist to determine if the original goal—such as reducing healthcare-associated infections—was met, facilitating continuous improvement.
References: CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.4 - Evaluate the effectiveness of infection prevention and control interventions; Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs.
An infection control manager is training a new infection preventionist. In discussing surveillance strategies, which of the following types of hospital infection surveillance usually provides maximum benefit with minimum resources?
High-risk patient focus
Antibiotic monitoring
Prevalence surveys
Nursing care plan review
Answer:
Explanation:
A high-risk patient focus maximizes benefits while minimizing resource use in infection surveillance.
Step-by-Step Justification:
Efficiency of High-Risk Surveillance:
Targeting ICU, immunocompromised patients, or surgical units helps detect infections where the risk is highest, leading to earlier interventions​.
Resource Allocation:
Full hospital-wide surveillance is resource-intensive; focusing on high-risk groups is more efficient.
Why Other Options Are Incorrect:
B. Antibiotic monitoring:
Important for stewardship, but not the primary focus of infection surveillance.
C. Prevalence surveys:
Snapshot data only; does not provide ongoing monitoring.
D. Nursing care plan review:
Less direct in identifying infection trends.
CBIC Infection Control References:
APIC Text, "Surveillance Strategies for Infection Prevention"​.
The infection preventionist (IP) is assisting pharmacists in investigating medication contamination at the hospital’s compounding pharmacy. As part of the medication recall process, the IP should:
Have laboratory culture all medication.
Inspect for safe injection practices.
Identify the potential source of contamination.
Inform all discharged patients of potential medication contamination.
Answer:
Explanation:
The scenario involves an infection preventionist (IP) assisting pharmacists in addressing medication contamination at the hospital’s compounding pharmacy, with a focus on the medication recall process. The IP’s role is to apply infection control expertise to mitigate risks, guided by the Certification Board of Infection Control and Epidemiology (CBIC) principles and best practices. The recall process requires a systematic approach to identify, contain, and resolve the issue, and the “first†or most critical step must be determined. Let’s evaluate each option:
A. Have laboratory culture all medication: Culturing all medication to confirm contamination is a valuable step to identify affected batches and guide the recall. However, this is a resource-intensive process that depends on first understanding the scope and source of the problem. Without identifying the potential source of contamination, culturing all medication could be inefficient and delay the recall. This step is important but secondary to initial investigation.
B. Inspect for safe injection practices: Inspecting for safe injection practices (e.g., single-use vials, proper hand hygiene, sterile technique) is a critical infection control measure, especially in compounding pharmacies where contamination often arises from procedural errors (e.g., reuse of syringes, improper cleaning). While this is a proactive step to prevent future contamination, it addresses ongoing practices rather than the immediate recall process for the current contamination event. It is a complementary action but not the first priority.
C. Identify the potential source of contamination: Identifying the potential source of contamination is the foundational step in the recall process. This involves investigating the compounding environment (e.g., water quality, equipment, personnel practices), raw materials, and production processes to pinpoint where the contamination occurred (e.g., bacterial ingress, cross-contamination). The CBIC emphasizes root cause analysis as a key infection prevention strategy, enabling targeted recalls, corrective actions, and prevention of recurrence. This step is essential before culturing, inspecting, or notifying patients, making it the IP’s primary responsibility in this context.
D. Inform all discharged patients of potential medication contamination: Notifying patients is a critical step to ensure public safety and allow for medical follow-up if they received contaminated medication. However, this action requires prior identification of the contaminated batches and their distribution, which depends on determining the source and confirming the extent of the issue. Premature notification without evidence could cause unnecessary alarm and is not the first step in the recall process.
The best answer is C, as identifying the potential source of contamination is the initial and most critical step in the medication recall process. This allows the IP to collaborate with pharmacists to trace the contamination, define the affected products, and guide subsequent actions (e.g., culturing, inspections, notifications). This aligns with CBIC’s focus on systematic investigation and risk mitigation in healthcare-associated infection events.
References:
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain III: Prevention and Control of Infectious Diseases, which includes identifying sources of contamination in healthcare settings.
CBIC Examination Content Outline, Domain V: Management and Communication, which emphasizes root cause analysis during outbreak investigations.
CDC Guidelines for Safe Medication Compounding (2022), which recommend identifying contamination sources as the first step in a recall process.
A nurse claims to have acquired hepatitis A virus infection as the result of occupational exposure. The source patient had an admitting diagnosis of viral hepatitis. Further investigation of this incident reveals a 5-day interval between exposure and onset of symptoms in the nurse. The patient has immunoglobulin G antibodies to hepatitis A. From the evidence, the infection preventionist may correctly conclude which of the following?
The nurse should be given hepatitis A virus immunoglobulin.
The evidence at this time fails to support the nurse's claim.
The patient has serologic evidence of recent hepatitis A viral infection.
The 5-day incubation period is consistent with hepatitis A virus transmission.
Answer:
Explanation:
The infection preventionist’s (IP) best conclusion, based on the provided evidence, is that the evidence at this time fails to support the nurse's claim of acquiring hepatitis A virus (HAV) infection through occupational exposure. This conclusion is grounded in the clinical and epidemiological understanding of HAV, as aligned with the Certification Board of Infection Control and Epidemiology (CBIC) guidelines. Hepatitis A typically has an incubation period ranging from 15 to 50 days, with an average of approximately 28-30 days, following exposure to the virus (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.3 - Apply principles of epidemiology). The reported 5-day interval between exposure and symptom onset in the nurse is significantly shorter than the expected incubation period, making it inconsistent with HAV transmission. Additionally, the presence of immunoglobulin G (IgG) antibodies in the source patient indicates past exposure or immunity to HAV, rather than an active or recent infection, which would typically be associated with immunoglobulin M (IgM) antibodies during the acute phase.
Option A (the nurse should be given hepatitis A virus immunoglobulin) is not supported because post-exposure prophylaxis with HAV immunoglobulin is recommended only within 14 days of exposure to a confirmed case with active infection, and the evidence here does not confirm a recent exposure or active case. Option C (the patient has serologic evidence of recent hepatitis A viral infection) is incorrect because IgG antibodies signify past infection or immunity, not a recent infection, which would require IgM antibodies. Option D (the 5-day incubation period is consistent with hepatitis A virus transmission) is inaccurate due to the mismatch with the known incubation period of HAV.
The IP’s role includes critically evaluating epidemiological data to determine the likelihood of transmission events. The discrepancy in the incubation period and the serologic status of the patient suggest that the nurse’s claim may not be substantiated by the current evidence, necessitating further investigation rather than immediate intervention or acceptance of the claim. This aligns with CBIC’s emphasis on accurate identification and investigation of infectious disease processes (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.2 - Investigate suspected outbreaks or exposures).
References: CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competencies 1.2 - Investigate suspected outbreaks or exposures, 1.3 - Apply principles of epidemiology.
Immediate use steam sterilization is NOT recommended for implantable items requiring immediate use because
the high temperature may damage the items.
chemical indicators may not be accurate at high temperatures.
results of biologic indicators are unavailable prior to use of the item.
the length of time is inadequate for the steam to penetrate the pack.
Answer:
Explanation:
The correct answer is C, "results of biologic indicators are unavailable prior to use of the item," as this is the primary reason immediate use steam sterilization (IUSS) is not recommended for implantable items requiring immediate use. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, IUSS is a process used for sterilizing items needed urgently when no other sterile options are available, typically involving a shortened cycle (e.g., flash sterilization). However, for implantable items—such as orthopedic hardware or prosthetic devices—ensuring absolute sterility is critical due to the risk of deep infection. Biologic indicators (BIs), which contain highly resistant spores to verify sterilization efficacy, require incubation (typically 24-48 hours) to confirm the kill, but IUSS does not allow time for BI results to be available before the item is used (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This lack of immediate verification poses a significant infection risk, making IUSS inappropriate for implants, as per AAMI ST79 standards.
Option A (the high temperature may damage the items) is a consideration for some heat-sensitive materials, but modern IUSS cycles are designed to minimize damage, and this is not the primary reason for the restriction on implants. Option B (chemical indicators may not be accurate at high temperatures) is incorrect, as chemical indicators (e.g., color-changing strips) are reliable at high temperatures and serve as an immediate check, though they are not a substitute for BIs. Option D (the length of time is inadequate for the steam to penetrate the pack) is not the main issue, as IUSS cycles are optimized for penetration, though the shortened time may be a secondary concern; the unavailability of BI results remains the decisive factor.
The focus on biologic indicator results aligns with CBIC’s emphasis on ensuring the safety and sterility of reprocessed medical devices, particularly for high-risk implantable items (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This recommendation is supported by AAMI and CDC guidelines, which prioritize BI confirmation for implants to prevent healthcare-associated infections (AAMI ST79:2017, CDC Sterilization Guidelines, 2019).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities. CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2019.
What is a characteristic of immediate-use steam sterilization?
Alternative to purchasing expensive instrument sets.
Can be used for the following surgery if properly stored.
Substitute for maintaining sufficient amounts of sterile instruments.
Performed in emergencies where cleaning is the most critical step.
Answer:
Explanation:
The correct answer is C, "Substitute for maintaining sufficient amounts of sterile instruments," as this is a characteristic of immediate-use steam sterilization (IUSS). According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, IUSS, formerly known as flash sterilization, is a process designed to rapidly sterilize items that are needed urgently when pre-sterilized inventory is unavailable or insufficient. It serves as a temporary solution to address gaps in sterile instrument availability, such as during unexpected surges in surgical demand or equipment shortages, provided strict protocols are followed (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). However, IUSS is not a routine practice and should be minimized due to its limitations, including the lack of immediate biologic indicator results.
Option A (alternative to purchasing expensive instrument sets) is incorrect because IUSS is not intended as a cost-saving measure or a replacement for acquiring necessary equipment; it is a contingency process. Option B (can be used for the following surgery if properly stored) is misleading, as IUSS items are intended for immediate use and not for storage or use in subsequent procedures, which requires standard sterilization cycles with proper packaging and validation. Option D (performed in emergencies where cleaning is the most critical step) overemphasizes cleaning and mischaracterizes IUSS; while cleaning is a critical initial step, the process is defined by its rapid sterilization for emergency use, not solely by cleaning priority.
The characteristic of substituting for insufficient sterile instruments aligns with CBIC’s focus on ensuring safe reprocessing practices while acknowledging the practical challenges in healthcare settings (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This is supported by AAMI ST79, which outlines IUSS as a last-resort measure to maintain surgical readiness (AAMI ST79:2017).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
Which of the following options describes a correct use of personal protective equipment?
Personal eyeglasses should be worn during suctioning.
Surgical masks should be worn during lumbar puncture procedures.
Gloves should be worn when handling or touching a cardiac monitor that has been disinfected.
Eye protection should be worn when providing patient care it at risk of spreading respiratory disease after unprotected exposure.
Answer:
Explanation:
According to CDC and APIC guidelines, a surgical mask is required when performing lumbar punctures to prevent bacterial contamination (e.g., meningitis caused by droplet transmission of oral flora).
Why the Other Options Are Incorrect?
A. Personal eyeglasses should be worn during suctioning – Incorrect because eyeglasses do not provide adequate eye protection. Goggles or face shields should be used.
C. Gloves should be worn when handling or touching a cardiac monitor that has been disinfected – Not necessary unless recontamination is suspected.
D. Eye protection should be worn when providing patient care after unprotected exposure – Eye protection should be used before exposure, not just after.
CBIC Infection Control Reference
APIC states that surgical masks must be worn for procedures such as lumbar puncture to reduce infection risk​.
A 2-yoar-old girl is admitted with a fractured tibia. At birth, she was diagnosed with congenital cytomegalovirus (CMV). Which of the following barrier precautions is appropriate for healthcare personnel caring for her?
Wear masks and gloves
Wear gloves when handling body fluids
No barrier precautions are needed
Use gowns, masks, gloves, and a private room
Answer:
Explanation:
Standard Precautions are sufficient for congenital cytomegalovirus (CMV), which means that gloves should be used when handling body fluids. CMV is primarily transmitted via direct contact with saliva, urine, or blood.
Why the Other Options Are Incorrect?
A. Wear masks and gloves – Masks are not necessary unless performing high-risk aerosol-generating procedures.
C. No barrier precautions are needed – Gloves are required when handling bodily fluids to prevent transmission.
D. Use gowns, masks, gloves, and a private room – CMV does not require Contact or Airborne Precautions.
CBIC Infection Control Reference
APIC guidelines state that CMV transmission is prevented using Standard Precautions, primarily with glove use for body fluid contact​.
During the past week, three out of four blood cultures from a febrile neonate in an intensive care unit grew coagulase-negative staphylococci. This MOST likely indicates:
Laboratory error.
Contamination.
Colonization.
Infection.
Answer:
Explanation:
The scenario involves a febrile neonate in an intensive care unit (ICU) with three out of four blood cultures growing coagulase-negative staphylococci (CoNS) over the past week. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes accurate interpretation of microbiological data in the "Identification of Infectious Disease Processes" domain, aligning with the Centers for Disease Control and Prevention (CDC) guidelines for healthcare-associated infections. Determining whether this represents a true infection, contamination, colonization, or laboratory error requires evaluating the clinical and microbiological context.
Option B, "Contamination," is the most likely indication. Coagulase-negative staphylococci, such as Staphylococcus epidermidis, are common skin flora and frequent contaminants in blood cultures, especially in neonates where skin preparation or sampling technique may be challenging. The CDC’s "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) and the Clinical and Laboratory Standards Institute (CLSI) note that multiple positive cultures (e.g., two or more) are typically required to confirm true bacteremia, particularly with CoNS, unless accompanied by clear clinical signs of infection (e.g., worsening fever, hemodynamic instability) and no other explanation. The inconsistency (three out of four cultures) and the neonate’s ICU setting—where contamination from skin or catheter hubs is common—suggest that the positive cultures likely result from contamination during blood draw rather than true infection. Studies, such as those in the Journal of Clinical Microbiology (e.g., Beekmann et al., 2005), indicate that CoNS in blood cultures is contaminated in 70-80% of cases when not supported by robust clinical correlation.
Option A, "Laboratory error," is possible but less likely as the primary explanation. Laboratory errors (e.g., mislabeling or processing mistakes) could occur, but the repeated growth in three of four cultures suggests a consistent finding rather than a random error, making contamination a more plausible cause. Option C, "Colonization," refers to the presence of microorganisms on or in the body without invasion or immune response. While CoNS can colonize the skin or catheter sites, colonization does not typically result in positive blood cultures unless there is an invasive process, which is not supported by the data here. Option D, "Infection," is the least likely without additional evidence. True CoNS bloodstream infections (e.g., catheter-related) in neonates are serious but require consistent positive cultures, clinical deterioration (e.g., persistent fever, leukocytosis), and often imaging or catheter removal confirmation. The febrile state alone, with inconsistent culture results, does not meet the CDC’s criteria for diagnosing infection (e.g., at least two positive cultures from separate draws).
The CBIC Practice Analysis (2022) and CDC guidelines stress differentiating contamination from infection to avoid unnecessary treatment, which can drive antibiotic resistance. Given the high likelihood of contamination with CoNS in this context, Option B is the most accurate answer.
References:
CBIC Practice Analysis, 2022.
CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2017.
Beekmann, S. E., et al. (2005). Coagulase-Negative Staphylococci in Blood Cultures. Journal of Clinical Microbiology.
CLSI Guidelines on Blood Culture Interpretation, 2018.
Which of the following processes is essential for endoscope reprocessing?
Intermediate level disinfection and contact time
Pre-cleaning, leak testing, and manual cleaning
Inspection using a borescope and horizontal storage
Leak testing, manual cleaning, and low level disinfection
Answer:
Explanation:
The correct answer is B, "Pre-cleaning, leak testing, and manual cleaning," as these processes are essential for endoscope reprocessing. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, proper reprocessing of endoscopes is critical to prevent healthcare-associated infections (HAIs), given their complex design and susceptibility to microbial contamination. The initial steps of pre-cleaning (removing gross debris at the point of use), leak testing (ensuring the endoscope’s integrity to prevent fluid ingress), and manual cleaning (using enzymatic detergents to remove organic material) are foundational to the reprocessing cycle. These steps prepare the endoscope for high-level disinfection or sterilization by reducing bioburden and preventing damage, as outlined in standards such as AAMI ST91 (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Failure at this stage can compromise subsequent disinfection, making it a non-negotiable component of the process.
Option A (intermediate level disinfection and contact time) is an important step but insufficient alone, as intermediate-level disinfection does not achieve the high-level disinfection required for semi-critical devices like endoscopes, which must eliminate all microorganisms except high levels of bacterial spores. Option C (inspection using a borescope and horizontal storage) includes valuable quality control (inspection) and storage practices, but these occur later in the process and are not essential initial steps; vertical storage is often preferred to prevent damage. Option D (leak testing, manual cleaning, and low level disinfection) includes two essential steps (leak testing and manual cleaning) but is inadequate because low-level disinfection does not meet the standard for endoscopes, which require high-level disinfection or sterilization.
The emphasis on pre-cleaning, leak testing, and manual cleaning aligns with CBIC’s focus on adhering to evidence-based reprocessing protocols to ensure patient safety and prevent HAIs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). These steps are mandated by guidelines to mitigate risks associated with endoscope use in healthcare settings.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.4 - Implement environmental cleaning and disinfection protocols. AAMI ST91:2015, Flexible and semi-rigid endoscope processing in health care facilities.
Which of the following processes is MOST important for the infection preventionist (IP) to review when evaluating a third-party reprocessor for single-use devices?
Observe all steps for reprocessing.
Review the facility's blueprints and policies.
Ensure air and water cultures are performed regularly.
Obtain feedback from other IPs who use the reprocessor.
Answer:
Explanation:
The correct answer is A, "Observe all steps for reprocessing," as this is the most important process for the infection preventionist (IP) to review when evaluating a third-party reprocessor for single-use devices. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the reprocessing of single-use devices (SUDs) by third-party entities must adhere to stringent infection control standards to ensure they are safe for reuse and do not contribute to healthcare-associated infections (HAIs). Observing all steps—such as cleaning, disinfection, sterilization, packaging, and quality control—allows the IP to directly assess compliance with manufacturer instructions, regulatory requirements (e.g., FDA guidelines), and best practices (e.g., AAMI ST91) (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This hands-on evaluation is critical because any deviation in the reprocessing chain can compromise device sterility and patient safety.
Option B (review the facility's blueprints and policies) provides context about the physical layout and procedural framework, but it is a preliminary step that does not directly verify the reprocessing process’s effectiveness. Option C (ensure air and water cultures are performed regularly) is important for monitoring environmental contamination risks, particularly in sterile processing areas, but it is a supportive measure rather than the primary focus of evaluating the reprocessor’s core activities. Option D (obtain feedback from other IPs who use the reprocessor) offers valuable peer insights, but it is subjective and secondary to direct observation, which provides firsthand evidence of compliance and performance.
The priority on observing reprocessing steps aligns with CBIC’s emphasis on ensuring the safety and efficacy of reprocessed medical devices, a key responsibility for IPs when outsourcing to third-party reprocessors (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This process enables the IP to identify specific weaknesses, validate adherence to standards, and make informed decisions about the reprocessor’s suitability.
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.3 - Ensure safe reprocessing of medical equipment, 3.5 - Evaluate the environment for infection risks. AAMI ST91:2015, Flexible and semi-rigid endoscope processing in health care facilities.
A family, including an infant of 8 months, is going on a vacation to Europe. An infection preventionist would recommend:
Exposure to rabies should be avoided.
Family members should be vaccinated for yellow fever.
The infant should not travel until at least 12 months of age.
Family immunization records should be reviewed by their provider.
Answer:
Explanation:
When advising a family, including an 8-month-old infant, planning a vacation to Europe, an infection preventionist (IP) must consider travel-related health risks and vaccination recommendations tailored to the destination and age-specific guidelines. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Education and Training" domain, which includes providing evidence-based advice to prevent infections, aligning with the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) travel health recommendations.
Option D, "Family immunization records should be reviewed by their provider," is the most appropriate recommendation. Europe, as a region, includes countries with varying health risks, but it is generally considered a low-risk area for many vaccine-preventable diseases compared to tropical regions. The CDC’s "Travelers’ Health" guidelines (2023) recommend that all travelers, including infants, have their immunization status reviewed by a healthcare provider prior to travel to ensure compliance with routine vaccinations (e.g., measles, mumps, rubella [MMR], diphtheria, tetanus, pertussis [DTaP], and polio) and to assess any destination-specific needs. For an 8-month-old, the review would confirm that the infant has received age-appropriate vaccines (e.g., the first doses of DTaP, Hib, PCV, and IPV, typically starting at 2 months) and is on schedule for the 6- and 12-month doses. This step ensures the family’s overall protection and identifies any gaps, making it a proactive and universally applicable recommendation.
Option A, "Exposure to rabies should be avoided," is a general travel safety tip applicable to any destination where rabies is endemic (e.g., parts of Eastern Europe or rural areas with wildlife). However, rabies risk in most European countries is low, and pre-exposure vaccination is not routinely recommended for travelers unless specific high-risk activities (e.g., handling bats) are planned. The CDC advises avoiding animal bites rather than vaccinating unless indicated, making this less specific and urgent than a records review. Option B, "Family members should be vaccinated for yellow fever," is incorrect. Yellow fever is not endemic in Europe, and vaccination is not required or recommended for travel to any European country. The WHO International Health Regulations (2005) and CDC list yellow fever vaccination as mandatory only for travelers from or to certain African and South American regions, rendering this irrelevant. Option C, "The infant should not travel until at least 12 months of age," lacks a clear evidence base. While some vaccines (e.g., MMR) are typically given at 12 months, the 8-month-old can travel safely if up-to-date on age-appropriate immunizations. The CDC allows travel for infants as young as 6 weeks with medical clearance, and delaying travel to 12 months is not a standard recommendation unless specific risks (e.g., disease outbreaks) are present, which are not indicated here.
The CBIC Practice Analysis (2022) and CDC Travelers’ Health resources prioritize pre-travel health assessments, including immunization reviews, as the foundation for safe travel. Option D ensures a comprehensive approach tailored to the family’s needs, making it the best recommendation for a trip to Europe.
References:
CBIC Practice Analysis, 2022.
CDC Travelers’ Health, 2023.
WHO International Health Regulations, 2005.
The correct answer is B, "Blood pressure cuff," as this item is appropriately cleaned with a disinfectant that is an approved hospital disinfectant with no tuberculocidal claim. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the selection of disinfectants for medical equipment depends on the item’s classification and intended use. The Environmental Protection Agency (EPA) categorizes hospital disinfectants based on their efficacy against specific pathogens, with tuberculocidal claims indicating effectiveness against Mycobacterium tuberculosis, a highly resistant organism. A disinfectant without a tuberculocidal claim is suitable for non-critical items—those that contact intact skin but not mucous membranes or sterile tissues—such as blood pressure cuffs, which require only low-level disinfection to reduce bacterial and viral loads (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). This aligns with CDC guidelines, which designate low-level disinfectants as adequate for non-critical surfaces.
Option A (laryngoscope blades) is incorrect because laryngoscope blades are semi-critical items that contact mucous membranes (e.g., the oropharynx) and require high-level disinfection or sterilization, which necessitates a disinfectant with tuberculocidal activity to ensure efficacy against a broader spectrum of pathogens, including mycobacteria. Option C (respiratory therapy equipment) is also incorrect, as this equipment (e.g., ventilators or nebulizers) is semi-critical or critical depending on its use, requiring at least intermediate- to high-level disinfection, which exceeds the capability of a non-tuberculocidal disinfectant. Option D (ultrasound probe) is inappropriate if used on intact skin (non-critical, allowing low-level disinfection), but many ultrasound probes contact mucous membranes or sterile sites, necessitating high-level disinfection with a tuberculocidal agent, making this option unreliable without context.
The selection of a blood pressure cuff aligns with CBIC’s emphasis on using appropriate disinfectants based on the Spaulding classification to prevent healthcare-associated infections (HAIs) (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.5 - Evaluate the environment for infection risks). This is supported by EPA and CDC guidelines, which guide disinfectant use based on item risk levels (EPA Disinfectant Product List, 2023; CDC Disinfection Guidelines, 2019).
References: CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competencies 3.4 - Implement environmental cleaning and disinfection protocols, 3.5 - Evaluate the environment for infection risks. EPA Disinfectant Product List, 2023. CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities, 2019.
An infection preventionist is informed that there is a possible cluster of streptococcal meningitis in the neonatal intensive care unit. Which of the following streptococcal serogroops is MOST commonly associated with meningitis in neonates beyond one week of age?
Group A
Group B
Group C
Group D
Answer:
Explanation:
Group B Streptococcus (Streptococcus agalactiae) is the most common cause of neonatal bacterial meningitis beyond one week of age.
Step-by-Step Justification:
Group B Streptococcus (GBS) and Neonatal Infections:
GBS is a leading cause of late-onset neonatal meningitis (occurring after 7 days of age)​.
Infection typically occurs through vertical transmission from the mother or postnatal exposure.
Neonatal Risk Factors:
Premature birth, prolonged rupture of membranes, and maternal GBS colonization increase risk​.
Why Other Options Are Incorrect:
A. Group A: Rare in neonates and more commonly associated with pharyngitis and skin infections.
C. Group C: Typically associated with animal infections and rarely affects humans.
D. Group D: Includes Enterococcus, which can cause neonatal infections but is not the most common cause of meningitis.
CBIC Infection Control References:
APIC Text, "Group B Streptococcus and Neonatal Meningitis"​.

